In Vitro Biophysics
Our program focuses on characterizing the ways in which neurons store new information during associative learning at the cellular and subcellular levels. Experiments focus on the hippocampus, a paleocortical region involved in transferring information during learning from the short- to long-term memory store. We make biophysical measurements from hippocampal brain slices taken from eyeblink-trained animals to define what ionic mechanisms underlie the changes in neuronal excitability recorded in the intact animal. We have observed conditioning-specific alterations in postsynaptic intrinsic currents (calcium activated potassium currents) likely to enhance cellular excitability and are performing current and whole-cell patch clamp recordings to characterize them and their relation to acquisition and consolidation of the eyeblink-conditioned response. An important focus of our research is on cellular mechanisms for altered learning in aging. We are using a combination of behavioral and biophysical approaches to address this question.
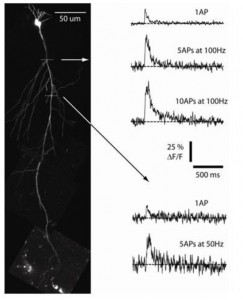
Recently, we have incorporated calcium-imaging techniques using both a charge-coupled device (CCD) camera system and a two-photon laser scanning microscopy (2P-LSM) system to investigate learning- and aging-related changes in calcium properties in CA1 pyramidal neurons. In addition, we have added the capacity to investigate calcium transients in dendritic spines by using two-photon glutamate uncaging. Thus, these latest, powerful tools will be used to complement our traditional biophysical measures in pursuing the cellular mechanism(s) that underlie learning- and aging-related changes in the hippocampal neurons.
Representative publications:
- Simkin D, Hattori S, Ybarra N, Musial TF, Buss EW, Richter H, Oh MM, Nicholson DA, Disterhoft JF. Aging-Related Hyperexcitability in CA3 Pyramidal Neurons Is Mediated by Enhanced A-Type K+ Channel Function and Expression. J Neurosci. 2015 Sep 23;35(38):13206-18. PubMed PMID: 26400949; PubMed Central PMCID: PMC4579378.
- Oh MM, Oliveira FA, Waters J, Disterhoft JF. Altered calcium metabolism in aging CA1 hippocampal pyramidal neurons. J Neurosci. 2013 May 1;33(18):7905-11. PubMed PMID: 23637181; PubMed Central PMCID:PMC3679661.
- McKay BM, Oh MM, Disterhoft JF. Learning increases intrinsic excitability of hippocampal interneurons. J Neurosci. 2013 Mar 27;33(13):5499-506. PubMed PMID: 23536065; PubMed Central PMCID: PMC3678545.
- Kaczorowski CC, Sametsky E, Shah S, Vassar R, Disterhoft JF. Mechanisms underlying basal and learning-related intrinsic excitability in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2011 Aug;32(8):1452-65. PubMed PMID: 19833411; PubMed Central PMCID: PMC2888747.
- Matthews EA, Linardakis JM, Disterhoft JF. The fast and slow afterhyperpolarizations are differentially modulated in hippocampal neurons by aging and learning. J Neurosci. 2009 Apr 15;29(15):4750-5. PubMed PMID: 19369544; PubMed Central PMCID: PMC2678237.
- Oh MM, McKay BM, Power JM, Disterhoft JF. Learning-related postburst afterhyperpolarization reduction in CA1 pyramidal neurons is mediated by protein kinase A. Proc Natl Acad Sci U S A. 2009 Feb 3;106(5):1620-5. PubMed PMID: 19164584; PubMed Central PMCID: PMC2635792.